Metrix COVID-19 Test Kit, Box of 25
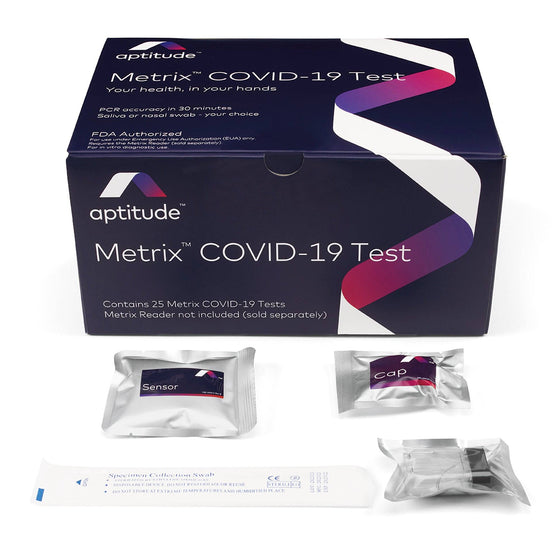
Metrix COVID-19 Test Kit, Box of 25
FREE SHIPPING
Features
- FDA Emergency Use Authorization
- Molecular diagnostic for SARS-CoV-2
- Home use with nasal swab or saliva
- Positive results indicate viral RNA presence
- Negative results need confirmation by molecular testing
- Requires Metrix Reader (sold separately)
Description
The Metrix COVID-19 Test is authorized under the FDA’s Emergency Use Authorization for the qualitative detection of SARS-CoV-2 nucleic acid. Designed for non-prescription home use, it accepts anterior nasal swab or saliva specimens collected by individuals aged 14 years and older or collected from children aged 2 years and older by adults. Positive results suggest viral RNA presence, necessitating clinical correlation for infection status. However, positive results do not exclude bacterial infection or co-infection with other viruses. Individuals testing positive should self-isolate and consult healthcare providers for further evaluation. Negative results from saliva require confirmation via alternative sample types if clinically indicated. The Metrix COVID-19 Test is the first over-the-counter molecular test authorized by the FDA for use with saliva or swab samples, requiring a Metrix Reader (sold separately) for result interpretation.
- FDA Emergency Use Authorization
- Molecular diagnostic for SARS-CoV-2
- Home use with nasal swab or saliva
- Positive results indicate viral RNA presence
- Negative results need confirmation by molecular testing
- Requires Metrix Reader (sold separately)
The Metrix COVID-19 Test is authorized under the FDA’s Emergency Use Authorization for the qualitative detection of SARS-CoV-2 nucleic acid. Designed for non-prescription home use, it accepts anterior nasal swab or saliva specimens collected by individuals aged 14 years and older or collected from children aged 2 years and older by adults. Positive results suggest viral RNA presence, necessitating clinical correlation for infection status. However, positive results do not exclude bacterial infection or co-infection with other viruses. Individuals testing positive should self-isolate and consult healthcare providers for further evaluation. Negative results from saliva require confirmation via alternative sample types if clinically indicated. The Metrix COVID-19 Test is the first over-the-counter molecular test authorized by the FDA for use with saliva or swab samples, requiring a Metrix Reader (sold separately) for result interpretation.
- FDA Emergency Use Authorization
- Molecular diagnostic for SARS-CoV-2
- Home use with nasal swab or saliva
- Positive results indicate viral RNA presence
- Negative results need confirmation by molecular testing
- Requires Metrix Reader (sold separately)
Description
The Metrix COVID-19 Test is authorized under the FDA’s Emergency Use Authorization for the qualitative detection of SARS-CoV-2 nucleic acid. Designed for non-prescription home use, it accepts anterior nasal swab or saliva specimens collected by individuals aged 14 years and older or collected from children aged 2 years and older by adults. Positive results suggest viral RNA presence, necessitating clinical correlation for infection status. However, positive results do not exclude bacterial infection or co-infection with other viruses. Individuals testing positive should self-isolate and consult healthcare providers for further evaluation. Negative results from saliva require confirmation via alternative sample types if clinically indicated. The Metrix COVID-19 Test is the first over-the-counter molecular test authorized by the FDA for use with saliva or swab samples, requiring a Metrix Reader (sold separately) for result interpretation.
| Item # | 192587 |
| MPN | MTRX-C19-25PK |
| Manufacturer | Sekisui Diagnostics |
| UOM Packaging | Box of 25 |
| UOM | BX |
Features
- FDA Emergency Use Authorization
- Molecular diagnostic for SARS-CoV-2
- Home use with nasal swab or saliva
- Positive results indicate viral RNA presence
- Negative results need confirmation by molecular testing
- Requires Metrix Reader (sold separately)
The Metrix COVID-19 Test is authorized under the FDA’s Emergency Use Authorization for the qualitative detection of SARS-CoV-2 nucleic acid. Designed for non-prescription home use, it accepts anterior nasal swab or saliva specimens collected by individuals aged 14 years and older or collected from children aged 2 years and older by adults. Positive results suggest viral RNA presence, necessitating clinical correlation for infection status. However, positive results do not exclude bacterial infection or co-infection with other viruses. Individuals testing positive should self-isolate and consult healthcare providers for further evaluation. Negative results from saliva require confirmation via alternative sample types if clinically indicated. The Metrix COVID-19 Test is the first over-the-counter molecular test authorized by the FDA for use with saliva or swab samples, requiring a Metrix Reader (sold separately) for result interpretation.