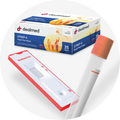
Veritor Covid and Influenza A&B Test Assay, Kit of 30

Veritor Covid and Influenza A&B Test Assay, Kit of 30
FREE SHIPPING
Features
- Rapid detection results
- Distinguishes between SARS-CoV-2, Flu A, and Flu B
- Individual digital display readouts
- Authorized by FDA under EUA for authorized laboratories
- Detects specific proteins via nasal swab sample
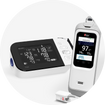
- For use with: BD Vertitor Plus Analyzer
- Sold as a 30 Test Kits
Description
The BD Veritor System offers a rapid and accurate method for detecting SARS-CoV-2, influenza A, and influenza B. With results available in about 15 minutes, the system provides individual digital display readouts for each virus.
This product has not been FDA cleared or approved, it has been authorized the FDA under an Emergency Use Authorization (EUA) for use in authorized laboratories. Please note that it is specifically designed for detecting proteins from SARS-CoV-2, influenza A, and influenza B, and its emergency use authorization is subject to the duration of the declaration justifying its use for COVID-19 detection and diagnosis.
Kit Includes:
- (30) Single Use Test Devices
- (30) Reaction Tubes with 400 µL Reagent
- (30) Sterile Single Use Sampling Swabs
- (1) Positive SARS-CoV-2 Control Swab
- (1) Positive Flu A Control Swab
- (1) Positive Flu B Control Swab
- (3) Paperboard Tube Stands
- Nasal Sampling Guide
- Quick Ref Card
- IFU
- Rapid detection results
- Distinguishes between SARS-CoV-2, Flu A, and Flu B
- Individual digital display readouts
- Authorized by FDA under EUA for authorized laboratories
- Detects specific proteins via nasal swab sample
- For use with: BD Vertitor Plus Analyzer
- Sold as a 30 Test Kits
The BD Veritor System offers a rapid and accurate method for detecting SARS-CoV-2, influenza A, and influenza B. With results available in about 15 minutes, the system provides individual digital display readouts for each virus.
This product has not been FDA cleared or approved, it has been authorized the FDA under an Emergency Use Authorization (EUA) for use in authorized laboratories. Please note that it is specifically designed for detecting proteins from SARS-CoV-2, influenza A, and influenza B, and its emergency use authorization is subject to the duration of the declaration justifying its use for COVID-19 detection and diagnosis.
Kit Includes:
- (30) Single Use Test Devices
- (30) Reaction Tubes with 400 µL Reagent
- (30) Sterile Single Use Sampling Swabs
- (1) Positive SARS-CoV-2 Control Swab
- (1) Positive Flu A Control Swab
- (1) Positive Flu B Control Swab
- (3) Paperboard Tube Stands
- Nasal Sampling Guide
- Quick Ref Card
- IFU
Features
- Rapid detection results
- Distinguishes between SARS-CoV-2, Flu A, and Flu B
- Individual digital display readouts
- Authorized by FDA under EUA for authorized laboratories
- Detects specific proteins via nasal swab sample
- For use with: BD Vertitor Plus Analyzer
- Sold as a 30 Test Kits
Description
The BD Veritor System offers a rapid and accurate method for detecting SARS-CoV-2, influenza A, and influenza B. With results available in about 15 minutes, the system provides individual digital display readouts for each virus.
This product has not been FDA cleared or approved, it has been authorized the FDA under an Emergency Use Authorization (EUA) for use in authorized laboratories. Please note that it is specifically designed for detecting proteins from SARS-CoV-2, influenza A, and influenza B, and its emergency use authorization is subject to the duration of the declaration justifying its use for COVID-19 detection and diagnosis.
Kit Includes:
- (30) Single Use Test Devices
- (30) Reaction Tubes with 400 µL Reagent
- (30) Sterile Single Use Sampling Swabs
- (1) Positive SARS-CoV-2 Control Swab
- (1) Positive Flu A Control Swab
- (1) Positive Flu B Control Swab
- (3) Paperboard Tube Stands
- Nasal Sampling Guide
- Quick Ref Card
- IFU
| Item # | 256088 |
| Manufacturer | BD |
| MPN | 256088 |
| UOM Packaging | Kit of 30 |
| UOM | KT |
| Disposable | Yes |
| Test Format | Test Device |
| Number of Tests | 30 Tests |
| CLIA | Clia Waived |
| Temp. Range | 2°C to 30°C |
| Reading Type | Machine Read |
| Test Type | Antigen Detection |
| Time to Results | 15 minutes |

| Item # | 256088 |
| MPN | 256088 |
| Manufacturer | BD |
| UOM Packaging | Kit of 30 |
| UOM | KT |
| Disposable | Yes |
| Test Format | Test Device |
| Number of Tests | 30 Tests |
| CLIA | Clia Waived |
| Temp. Range | 2°C to 30°C |
| Reading Type | Machine Read |
| Test Type | Antigen Detection |
| Time to Results | 15 minutes |
Features
- Rapid detection results
- Distinguishes between SARS-CoV-2, Flu A, and Flu B
- Individual digital display readouts
- Authorized by FDA under EUA for authorized laboratories
- Detects specific proteins via nasal swab sample
- For use with: BD Vertitor Plus Analyzer
- Sold as a 30 Test Kits
The BD Veritor System offers a rapid and accurate method for detecting SARS-CoV-2, influenza A, and influenza B. With results available in about 15 minutes, the system provides individual digital display readouts for each virus.
This product has not been FDA cleared or approved, it has been authorized the FDA under an Emergency Use Authorization (EUA) for use in authorized laboratories. Please note that it is specifically designed for detecting proteins from SARS-CoV-2, influenza A, and influenza B, and its emergency use authorization is subject to the duration of the declaration justifying its use for COVID-19 detection and diagnosis.
Kit Includes:
- (30) Single Use Test Devices
- (30) Reaction Tubes with 400 µL Reagent
- (30) Sterile Single Use Sampling Swabs
- (1) Positive SARS-CoV-2 Control Swab
- (1) Positive Flu A Control Swab
- (1) Positive Flu B Control Swab
- (3) Paperboard Tube Stands
- Nasal Sampling Guide
- Quick Ref Card
- IFU
Specifications
Product Specs
| Item # | 256088 |
| Manufacturer | BD |
| Color | - |
| Latex-Free | - |
| UOM | KT |
| Ply | - |
| UOM | KT |
| MPN | 256088 |
| Size | - |
| Sterile | - |
| UOM Packaging | Kit of 30 |
| Country of Origin | - |
| Woven | - |